-
Phase Transitions in Mesostructured Silica/Surfactant Composites: Surfactant Packing and the Role of Charge Density Matching
S.H. Tolbert, C.C. Landry, G.D. Stucky, B.F. Chmelka, P. Norby, J.C. Hanson and A. Monnier
Chemistry of Materials, 13 (7) (2001), p2247-2256


DOI:10.1021/cm0003727 | unige:3216 | Abstract | Article HTML | Article PDF
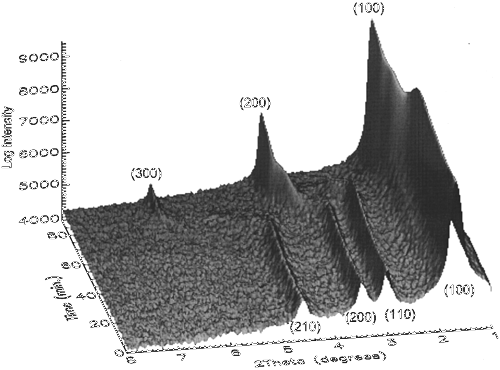
Time-resolved X-ray diffraction is utilized to follow phase transitions in nanostructured silica/surfactant composites in real time under hydrothermal conditions. The data allow us both to obtain kinetic parameters and to observe intermediate phases. In all cases, changes in the packing of the organic component of these composites drives the transformation, indicating that surfactant packing is a dominant factor in determining the overall structure of these materials. For materials heated in pure water, however, high activation energies for transformation were measured, suggesting that large kinetic barriers can stabilize structures against surfactant-driven rearrangements. Matching between the interfacial charge density of the inorganic silica framework and the charge density of the surfactant headgroups is also found to affect the kinetics of transformation. Lamellar-to-hexagonal transitions, which complement condensation-induced changes in charge density, are observed to be continuous, while hexagonal-to-lamellar transitions, which proceed contrary to these charge density changes, are discontinuous. For materials heated in their high-pH synthesis solutions, more complex phase behaviors are observed. Hexagonal (p6mm) structures transform either to a bicontinuous cubic phase (Ia3d) or to a lamellar structure. Lamellar phases are observed at either long or short polymerization times, while cubic phases dominate at intermediate polymerization times. The production of these different phases can be understood by considering the interplay between organic packing, charge density matching, and changing activation energies. At short times, high charge on the inorganic framework favors transformation to the low-curvature lamellar structures. At very long times, silica condensation both reduces this charge density and cross-links the framework. This cross-linking raises kinetic barriers for transformation and again favors the topologically simpler hexagonal-to-lamellar transition. Transformations to the cubic phase are only observed at intermediate times, when these effects are balanced.
